Routine viral respiratory testing.
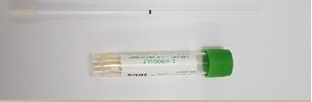
Specimen Volume
Viral transport mediaSpecimen Transport
Sample must be sent to the laboratory within 3 hours of collectionTurnaround Time
Within 24 hoursSample Stability
If transport is delayed, sample can be refrigerated for a maximum of 12 hoursGeneral Information
Nose/throat / nasopharyngeal /oropharyngeal swabs for this test must be received in 1ml or 3ml viral transport media.
For routine viral respiratory testing, the following viruses will be tested using the Hologic Fusion assay:
- Sars-CoV-2
- Influenza A
- Influenza B
- RSV A/B
An extended respiratory panel is available for Haematology and transplant patients which includes these additional viruses:
- Adenovirus
- Rhinovirus
- Human Metapneumovirus
- Parainfluenza 1,2,3,4
If a comprehensive respiratory virus screen is required, e.g. for CCU immuno-compromised patients, please refer to BioFire Filmarray Respiratory Panel.
If you are unsure; contact the laboratory using extension number 16534 during normal working hours or via switchboard at other times.
Results: are communicated electronically to Bed Management Teams and Infection Control.
Information Sharing: If your sample needs to be referred or a notifiable disease is detected from your sample, your details and sample may be shared with the UK Health Security Agency (UKHSA). This is a legal requirement. The UKHSA is sponsored by the Department of Health and Social Care.
Accreditation: This test is UKAS accredited to ISO15189:2022
Specifications
- EQA Status: Enrolled to UK NEQAS scheme
- EQAS Scheme: Yes